
EFFICACY & SAFETY
MONOFERRIC VS FERRIC CARBOXYMALTOSE
THE PHOSPHARE TRIALS REPORTED A SIGNIFICANTLY LOWER INCIDENCE OF HYPOPHOSPHATEMIA WITH MONOFERRIC VS FERRIC CARBOXYMALTOSE
Two identical, randomized (1:1), open-label, comparative trials in 245 patients total across two trials with IDA and intolerance or unresponsiveness to oral iron. Monoferric was administered as a single dose of 1000 mg infused over 20 minutes on Day 0. Ferric carboxymaltose was administered at 750 mg on Day 0 and 750 mg on Day 7. See full study design below.
Pooled incidence of hypophosphatemia (<2.0 mg/dL) in the Phosphare Trials (primary endpoint)5
Percentage of patients with hypophosphatemia from baseline to Day 35:
- Trial A: 7.9% with Monoferric vs 75.0% with ferric carboxymaltose [adjusted rate difference, –67.0% {95% CI, –77.4% to –51.5%}]
- Trial B: 8.1% with Monoferric vs 73.7% with ferric carboxymaltose [adjusted rate difference, –65.8% {95% CI, –76.6% to –49.8%}]
- Pooled Data: 8.0% with Monoferric vs 74.4% with ferric carboxymaltose [adjusted rate difference, –68.3% {95% CI, –76.5% to –56.5%}]
Post Hoc Analysis: Pooled incidence of severe hypophosphatemia (≤1.0 mg/dL)
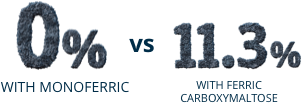
Post Hoc Analysis:
Pooled incidence
of severe hypophosphatemia (≤1.0 mg/dL)

Percentage of patients
with hypophosphatemia
from baseline to Day 35:
- Trial A: 7.9% with Monoferric vs 75.0% with ferric carboxymaltose [adjusted rate difference, –67.0% {95% CI, –77.4% to –51.5%}]
- Trial B: 8.1% with Monoferric vs 73.7% with ferric carboxymaltose [adjusted rate difference, –65.8% {95% CI, –76.6% to –49.8%}]
- Pooled Data: 8.0% with Monoferric vs 74.4% with ferric carboxymaltose [adjusted rate difference, –68.3% {95% CI, –76.5% to –56.5%}]
Study limitations
The 2 trials enrolled mostly women with iron-deficiency anemia due to gynecological bleeding, who tend to have higher rates of hypophosphatemia. This likely explains the higher than anticipated incidence of hypophosphatemia following ferric carboxymaltose treatment. Trials did not measure clinical outcome.
PHOSPHARE
Study Design
SEE STUDY +
CLOSE STUDY ^
SEE THE FULL
PHOSPHARE STUDY
READ FULL TEXT
See Monoferric Full Prescribing Information
PHOSPHARE-IBD: A HEAD-TO-HEAD STUDY IN IDA PATIENTS WITH INFLAMMATORY BOWEL DISEASE
This randomized, double-blind clinical study was conducted at 20 outpatient hospital clinics in Europe (Austria, Denmark, Germany, Sweden, and the UK). Ninety-seven adults with inflammatory bowel disease (IBD) and IDA were randomized 1:1 to receive Monoferric (n=49) or ferric carboxymaltose (FCM) (n=48) at baseline and at Day 35 using identical hemoglobin- and weight-based dosing regimens. PHOSPHARE-IBD patients were dosed with 1000 mg of Monoferric or FCM on Day 0. Then at Day 35, patients received either 1000 mg or 500 mg of Monoferric or 1000 mg or 500 mg of FCM. This is per the EMA label for both products.*
*Monoferric US dosing for patients ≥ 50 kg is 1,000 mg, which may be repeated if IDA reoccurs.
†Based on weight and Iron need at screening.
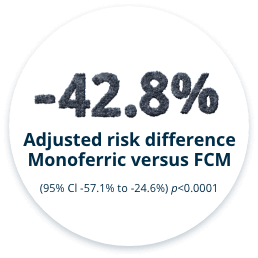
Primary Endpoint
- Incidence of hypophosphatemia (serum phosphate <2.0 mg/dL) from baseline to Day 35
Select Additional Endpoints
- Change in Hb concentrations from baseline to Day 70
- Change in FACIT-fatigue score from baseline to Day 70
- Change in biomarkers of bone and mineral metabolism from baseline to Day 70
- Incidence of adverse events and serious adverse events at any time during the study
The PHOSPHARE-IBD study evaluated patients through Day 70; however data are only presented through Day 35, as is consistent with the FDA-approved dosage in the US. For additional information on data beyond Day 35, please contact Medical Information at 888-828-0655 or MEDINFO@PHARMACOSMOS.US.
THE INCIDENCE OF HYPOPHOSPHATEMIA WAS SIGNIFICANTLY LESS WITH MONOFERRIC VS FCM FROM BASELINE TO DAY 35 (PRIMARY ENDPOINT)6



HEMOGLOBIN CHANGE, FACIT-FATIGUE SCORES, AND PHOSPHATE LEVELS (ADDITIONAL ENDPOINTS ARE NOT POWERED FOR SUPERIORITY)6,‡§
Change in Hb from Baseline to Day 35
Change in FACIT-Fatigue Score from
Baseline to Day 35
Change in S-Phosphate from
Baseline to Day 35
‡The PHOSPHARE-IBD study evaluated patients through Day 70; however, data are only presented through Day 35, as is consistent with US labeling.
§FACIT-Fatigue and Hb change graphs use the ITT population while S-Phosphate uses the safety analysis set.
SAFETY FROM PHOSPHARE-IBD STUDY6
Overall, adverse events (AEs) and serious adverse events (SAEs) occurred with comparable frequency in the Monoferric and FCM groups. Hypophosphatemia and vitamin D deficiency were reported as an AE more often in the FCM group than in the Monoferric group. Headache and nausea were reported as an AE more often in the Monoferric group than in the FCM group.
Adverse Events (≥10%) and Serious Adverse Events
HAVE A QUESTION ABOUT MONOFERRIC?
Sign up to contact a representative and receive information about Monoferric.
LET US HELP
SEE MONOFERRIC
VS IRON SUCROSE
Safety and efficacy evaluated in two randomized, open-label, non-inferiority comparative pivotal trials.1,3
REVIEW THE DATA
HOW MONOFERRIC WORKS
Monoferric has an innovative matrix structure that enables a slow and controlled release of bioavailable iron.
LEARN ABOUT IRON MATRIX STRUCTURE
INDICATIONS
Monoferric is indicated for the treatment of iron deficiency anemia (IDA) in adult patients:
- who have intolerance to oral iron or have had unsatisfactory response to oral iron
- who have non-hemodialysis dependent chronic kidney disease (NDD-CKD)
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Monoferric is contraindicated in patients with a history of serious hypersensitivity to Monoferric or any of its components. Reactions have included shock, clinically significant hypotension, loss of consciousness, and/or collapse.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving Monoferric. Patients may present with shock, clinically significant hypotension, loss of consciousness, and/or collapse. Monitor patients for signs and symptoms of hypersensitivity during and after Monoferric administration for at least 30 minutes and until clinically stable following completion of the infusion. Only administer Monoferric when personnel and therapies are immediately available for the treatment of serious hypersensitivity reactions. Monoferric is contraindicated in patients with prior serious hypersensitivity reactions to Monoferric or any of its components. In clinical trials in patients with IDA and CKD, serious or severe hypersensitivity were reported in 0.3% (6/2008) of the Monoferric treated subjects. These included 3 events of hypersensitivity in 3 patients; 2 events of infusion-related reactions in 2 patients and 1 event of asthma in one patient.
Iron Overload
Excessive therapy with parenteral iron can lead to excess iron storage and possibly iatrogenic hemosiderosis or hemochromatosis. Monitor the hematologic response (hemoglobin and hematocrit) and iron parameters (serum ferritin and transferrin saturation) during parenteral iron therapy. Do not administer Monoferric to patients with iron overload.
ADVERSE REACTIONS
Adverse reactions were reported in 8.6% (172/2008) of patients treated with Monoferric. Adverse reactions related to treatment and reported by ≥1% of the treated patients were nausea (1.2%) and rash (1%). Adjudicated serious or severe hypersensitivity reactions were reported in 6/2008 (0.3%) patients in the Monoferric group. Hypophosphatemia (serum phosphate <2.0 mg/dL) was reported in 3.5% of Monoferric-treated patients in Trials 1 & 2.
To report adverse events, please contact Pharmacosmos at 1-888-828-0655. You may also contact the FDA at www.fda.gov/medwatch or 1-800-FDA-1088.
References:
- Auerbach M, Henry D, Derman RJ, Achebe MM, Thomsen LL, Glaspy J. Am J Hematol. 2019;94(9):1007‐1014.
- Data on File. 2021. Pharmacosmos Therapeutics Inc.
- Bhandari S, Kalra PA, Berkowitz M, Belo D, Thomsen LL, Wolf M. Nephrol Dial Transplant. 2021;36(1):111-120.
- Monoferric (ferric derisomaltose) Prescribing Information; Pharmacosmos Therapeutics Inc., Morristown, NJ: 2022.
- Wolf M, Rubin J, Achebe M, et al. JAMA. 2020;323(5):432‐443.
- Zoller H, Wolf M, Blumenstein I, et al. Gut. 2022;0-10. doi:10.1136/gutjnl-2022-327897.